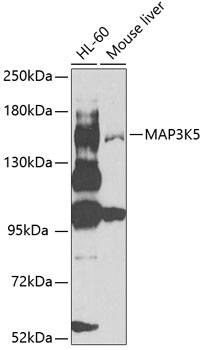
Apoptosis signal-regulating kinase 1 (MAP3K5), a MAP kinase kinase kinase, plays essential roles in stress-induced apoptosis (1,2). MAP3K5 is activated in response to a variety of stress-related stimuli through distinct mechanisms and activates MKK4 and MKK3, which in turn activate JNK and p38 (3). Overexpression of MAP3K5 activates JNK and p38 and induces apoptosis in several cell types through signals involving the mitochondrial cell death pathway. Embryonic fibroblasts or primary neurons derived from MAP3K5-/- mice are resistant to stress-induced JNK and p38 activation and cell death (4,5). Phosphorylation at Ser967 is essential for MAP3K5 association with 14-3-3 protein and suppression of cell death (6). Oxidative stress induces dephosphorylation of Ser967 and phosphorylation of Thr845 in the activation loop of MAP3K5, and both are correlated with MAP3K5 activity and MAP3K5-dependent apoptosis (7,8). On the other hand, Akt phosphorylates MAP3K5 at Ser83, which attenuates MAP3K5 activity and promotes cell survival (9).