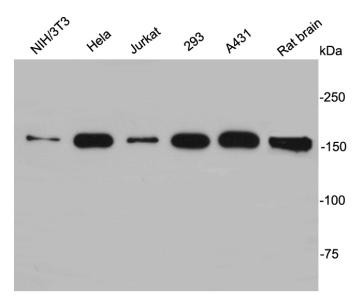
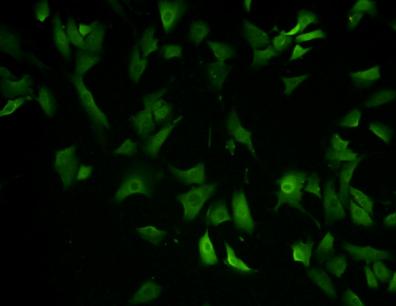
EEA1 localizes exclusively to early endosomes and has an important role in endosomal trafficking. EEA1 binds directly to the phospholipid phosphatidylinositol 3-phosphate through its C-terminal FYVE domain and forms a homodimer through a coiled coil . EEA1 acts as a tethering molecule that couples vesicle docking with SNAREs such as N-ethylmaleimide sensitive fusion protein, bringing the endosomes physically closer and ultimately resulting in the fusion and delivery of endosomal cargo. Due to the proteins importance in vesicular trafficking, a number of intracellular bacteria prevent EEA1 recruitment to the vacuole. Mycobacterium tuberculosis is known to inhibit the recruitment of EEA1 to the phagosomal membrane through CamKII.