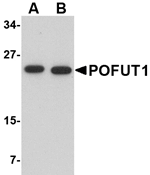
POFUT1, an endoplasmic reticulum-residing member of the glycosyltransferase O-Fuc family, adds O-fucose through an O-glycosidic linkage to conserved serine or threonines in epidermal growth factor-like repeats of several cell surface and secreted proteins. Unlike its homolog POFUT2, POFUT1 can also catalyze the transfer of fucose to thrombospondin type 1 repeats. Many of the substrates of POFUT1 are involved in ligand-induced receptor signaling. One such protein is Notch; mouse ES cells lacking POFUT have normal levels of Notch receptors at the cell surface, but these receptors do not bind Notch ligands or exhibit Notch signaling.