Product Detail
Product NameActive Caspase-3 Rabbit mAb
Clone No.SR01-02
Host SpeciesRecombinant Rabbit
Clonality Monoclonal
PurificationProA affinity purified
ApplicationsWB, ICC/IF, IHC
Species ReactivityHu
Immunogen Descrecombinant protein
ConjugateUnconjugated
Other NamesApopain antibody
CASP 3 antibody
CASP-3 antibody
CASP3 antibody
CASP3_HUMAN antibody
Caspase 3 antibody
Caspase-3 subunit p12 antibody
CPP 32 antibody
CPP-32 antibody
CPP32B antibody
Cysteine protease CPP32 antibody
PARP cleavage protease antibody
Protein Yama antibody
SCA-1 antibody
SCA1 antibody
SREBP cleavage activity 1 antibody
Yama antibody
Accession NoSwiss-Prot#:P42574
Uniprot
P42574
Gene ID
836;
Calculated MW17 kDa
Formulation1*TBS (pH7.4), 1%BSA, 40%Glycerol. Preservative: 0.05% Sodium Azide.
StorageStore at -20˚C
Application Details
WB: 1:1,000-1:2,000
IHC: 1:50-1:200
ICC: 1:50-1:200
Western blot analysis of active Caspase-3 on different cell lysates using anti-active Caspase-3 antibody at 1/1,000 dilution. Positive control:
Lane 1: Camptothecin (2 μM) treated Jurkat cells
Lane 2: Untreated Jurkat cells
Immunohistochemical analysis of paraffin-embedded human tonsil tissue using anti-active Caspase-3 antibody. Counter stained with hematoxylin.
Immunohistochemical analysis of paraffin-embedded human colon cancer tissue using anti-active Caspase-3 antibody. Counter stained with hematoxylin.
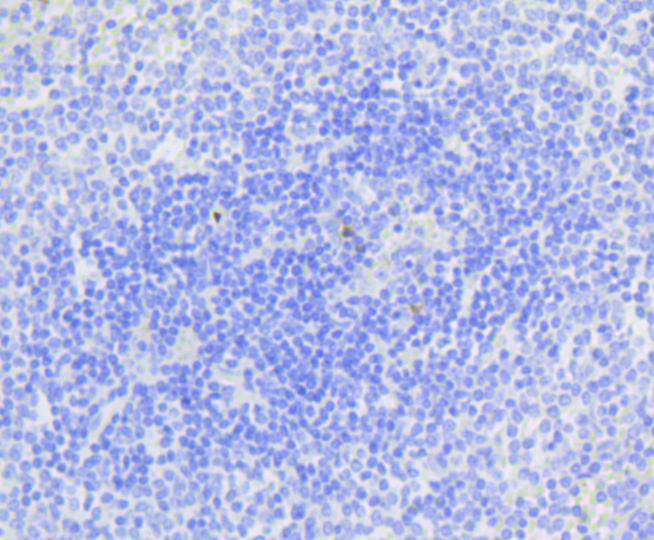
Immunohistochemical analysis of paraffin-embedded human spleen tissue using anti-active Caspase-3 antibody. Counter stained with hematoxylin.
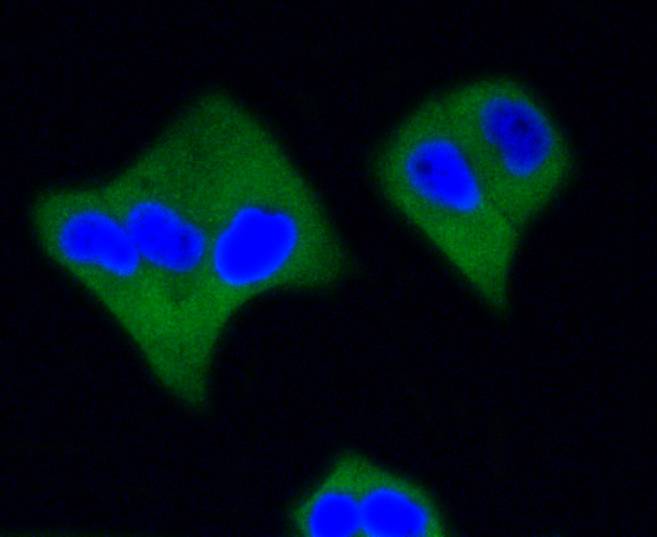
ICC staining active Caspase-3 in Hela cells (green). The nuclear counter stain is DAPI (blue). Cells were fixed in paraformaldehyde, permeabilised with 0.25% Triton X100/PBS.
ICC staining active Caspase-3 in PC-3M cells (green). The nuclear counter stain is DAPI (blue). Cells were fixed in paraformaldehyde, permeabilised with 0.25% Triton X100/PBS.
Caspase-3, also known as apopain, SCA-1, Yama and CPP32, is an aspartate-specific cysteine protease that belongs to the ICE subfamily of caspases. Caspase-3 is expressed in cells as an inactive precursor from which the p17 and p11 subunits of the mature caspase-3 are proteolytically generated during apoptosis. The caspase-3 precursor is first cleaved at Asp175-Ser176 to produce the p11 subunit and the p20 peptide. Subsequently, the p20 peptide is cleaved at Asp28-Ser29 to generate the mature p17 subunit. The active caspase-3 enzyme is a heterodimer composed of two p17 and two p11 subunits. At the onset of apoptosis, caspase-3 proteolytically cleaves PARP at an Asp216-Gly217 bond. During the execution of the apoptotic cascade, activated caspase-3 releases SREBP from the membrane of the ER in a proteolytic reaction that is distinct from their normal sterol-dependent activation. Caspase-3 cleaves and activates SREBPs between the basic helix-loop-helix leucine zipper domain and the membrane attachment domain. Caspase-3 also cleaves and activates caspase-6, -7 and -9. The human caspase-3 gene encodes a cytoplasmic protein that is highly expressed in lung, spleen, heart, liver, kidney and cells of the immune system.
If you have published an article using product 48667, please notify us so that we can cite your literature.






 Yes
Yes



