2. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis.
Yang W, Xia Y, Hawke D, Li X, Liang J, Aldape K, Hunter T, Yung WK, and Lu Z.
Cell 150(4):685-696, 8/2012.
PMID: 22901803
DOWNLOAD:

Summary:
Tumor-specific pyruvate kinase M2 (PKM2) is essential for the Warburg effect. In addition to its wellestablished role in aerobic glycolysis, PKM2 directly regulates gene transcription. However, the mechanism underlying this nonmetabolic function of PKM2 remains elusive. We show here that PKM2 directly binds to histone H3 and phosphorylates histone H3 at T11 upon EGF receptor activation. This phosphorylation is required for the dissociation of HDAC3 from the CCND1 and MYC promoter regions and subsequent acetylation of histone H3 at K9. PKM2-dependent histone H3 modifications are instrumental in EGF-induced expression of cyclin D1 and c-Myc, tumor cell proliferation, cell-cycle progression, and brain tumorigenesis. In addition, levels of histone H3 T11 phosphorylation correlate with nuclear PKM2 expression levels, glioma malignancy grades, and prognosis. These findings highlight the role of PKM2 as a protein kinase in its nonmetabolic functions of histone modification, which is essential for its epigenetic regulation of gene expression and tumorigenesis.
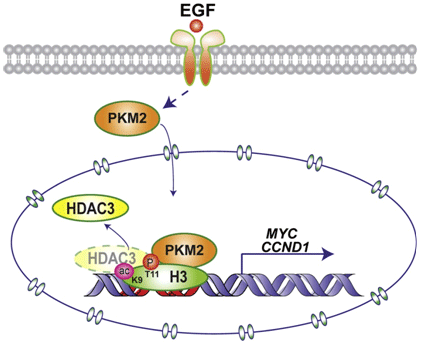
Figure 2: PKM2 Regulates Gene Expression by H3-T11 Phosphorylation
3. EGFR-induced and PKCε monoubiquitylation-dependent NF-κB activation upregulates PKM2 expression and promotes turmorigenesis
Yang W, Xia Y, Cao Y, Zheng Y, Bu W, Zhang L, You M, Koh M, Cote G, Aldape L. Li Y, Verma I, Chiao P, and Lu Z.
Molecular Cell 48(5):771-84, 2012.
PMID: 23123196
DOWNLOAD:

Summary:
Many types of human tumor cells have overexpressed pyruvate kinase M2 (PKM2). However, themechanism underlying this increased PKM2 expression remains to be defined. We demonstratehere that EGFR activation induces PLCγ1-dependent PKCε monoubiquitylation at Lys321mediated by RINCK1 ubiquitin ligase. Monoubiquitylated PKCε interacts with a ubiquitinbindingdomain in NEMO zinc finger and recruits the cytosolic IKK complex to the plasmamembrane, where PKCε phosphorylates IKKβ at Ser177 and activates IKKβ. Activated RelAinteracts with HIF1α, which is required for RelA to bind the PKM2 promoter. PKCε- and NF-κBdependentPKM2 upregulation is required for EGFR-promoted glycolysis and tumorigenesis. In addition, PKM2 expression correlates with EGFR and IKKβ activity in human glioblastomaspecimens and with grade of glioma malignancy. These findings highlight the distinct regulationof NF-κB by EGF, in contrast to TNFα, and the importance of the metabolic cooperation betweenthe EGFR and NF-κB pathways in PKM2 upregulation and tumorigenesis.

Figure 3: A mechanism for EGFR-induced PKM2 upregulation
4. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Fang G, Lyssiotis C, Aldape K, Cantley L, and Lu Z.
Nature Cell Biology 14(12):1295-304, 2012.
PMID: 23178880
DOWNLOAD:

Summary:
Pyruvate kinase M2 (PKM2) is upregulated in multiple cancer types and contributes to theWarburg effect by unclarified mechanisms. Here we demonstrate that EGFR-activated ERK2binds directly to PKM2 I429/L431 via the ERK2 docking groove and phosphorylates PKM2 Ser37but not PKM1. Phosphorylated PKM2 Ser37 recruits PIN1 for cis-trans isomerization of PKM2,which leads to PKM2 binding to importin α5 and nuclear translocation. Nuclear PKM2, acting asa coactivator of β-catenin, induces c-Myc expression, resulting in the upregulation of GLUT1,LDHA, and, in a positive feedback loop, PTB-dependent PKM2 expression. Replacement of wildtype PKM2 with a nuclear translocation-deficient mutant (S37A) blocks the EGFR-promotedWarburg effect and brain tumor development. In addition, levels of PKM2 S37 phosphorylationcorrelate with EGFR and ERK1/2 activity in human glioblastoma specimens. Our findingshighlight the importance of nuclear functions of PKM2 in the Warburg effect and tumorigenesis.
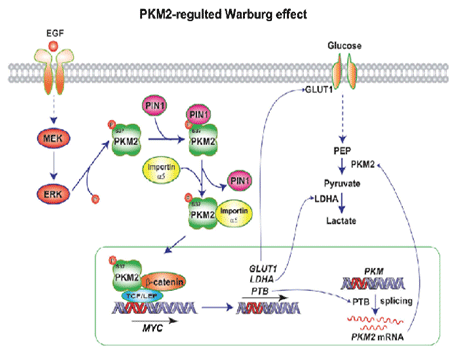
Figure 4: A mechanism of PKM2-regulated Warburg effect
© 2005-2020, Signalway Antibody Co.,Ltd. All Rights Reserved. E-mail: info@sabbiotech.com



